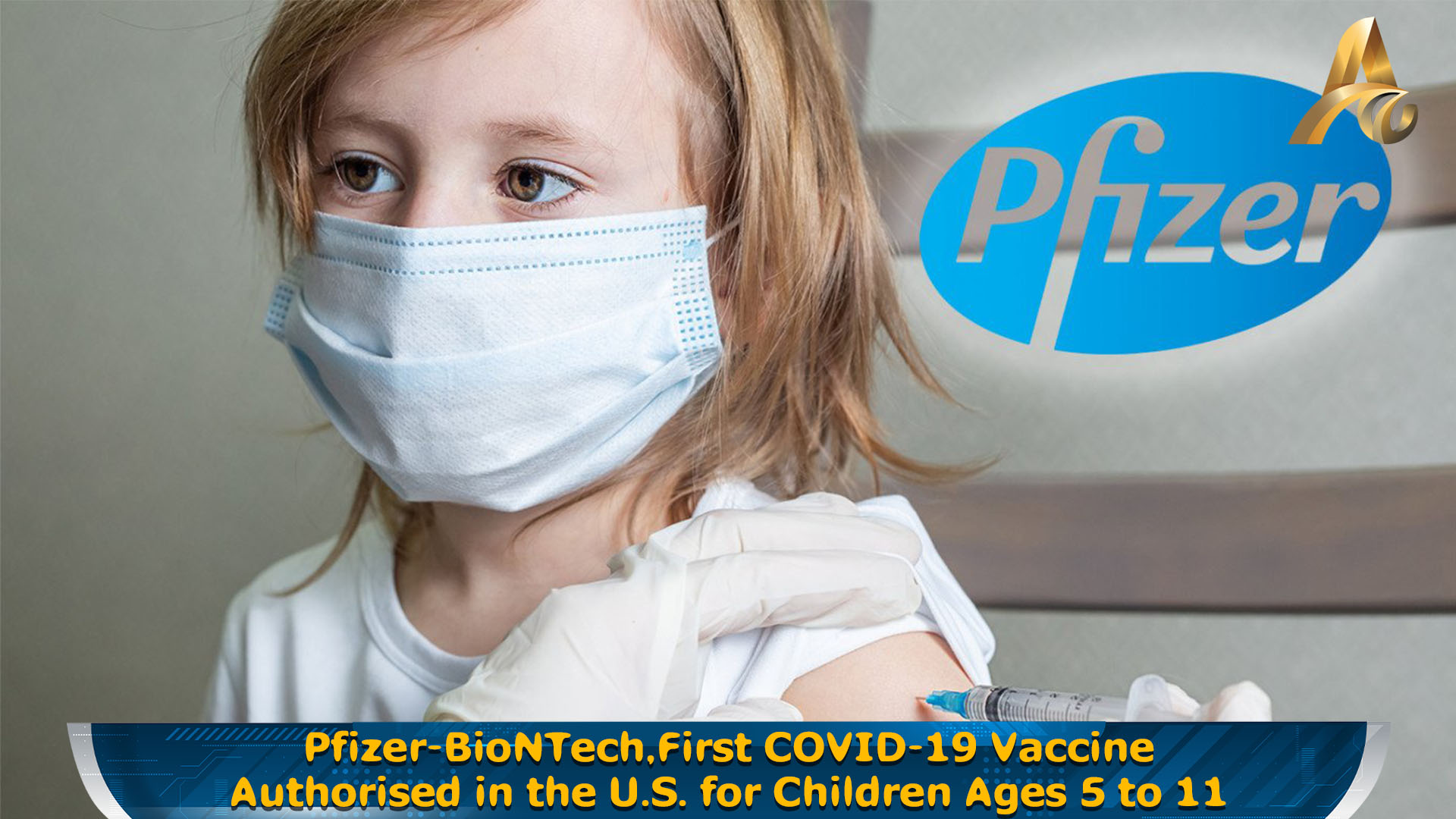
INTERNATIONAL: Pfizer and BioNTech has announced that the U.S. Food and Drug Administration (FDA) has authorized for emergency use the Pfizer-BioNTech COVID-19 Vaccine for children 5 through 11 years of age. For this age group, the vaccine is to be administered in a two-dose regime, given 21 days apart. This is the first COVID-19 vaccine authorized in the U.S. for individuals 5 through 11 years of age.
The emergency use authorization is supported by clinical data showing a favorable safety profile and high vaccine efficacy in children, underlining its potential to address a current public health need. This is assured by CEO and Co-founder of BioNTech, Ugur Sahin.
As a next step, the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) will meet next week to discuss a potential recommendation for the use and rollout of the vaccine to children 5 through 11 years of age. Pediatric vaccinations are anticipated to start, subject to, and after, CDC endorses the ACIP recommendation.
Pfizer and BioNTech have submitted requests for authorization of their COVID-19 vaccine in this age group to other regulators around the world, including the European Medicines Agency.
Pfizer has confirmed that it will begin shipping pediatric vials of the vaccine on Saturday to pharmacies, pediatricians' offices and other places where the shots may be administered.The FDA decision is expected to make the vaccine available to 28 million American children, many of whom are back in school for in-person learning. It comes after a panel of advisers to the regulator voted overwhelmingly to recommend the authorization on Tuesday.
Only a few other countries, including China, Cuba and the United Arab Emirates, have so far cleared COVID-19 vaccines for children in this age group and younger.
In the United States, around 58% of the population is fully vaccinated, lagging other nations such as the UK and France. Many adults, who have been hesitant to get a vaccine, may be more cautious about giving the shot to their children.
Pfizer and BioNTech says that their vaccine showed 90.7% efficacy against the coronavirus in a clinical trial of children aged 5 to 11. The United States started administering the vaccine to teens between ages 12 and 17 in May. Vaccination coverage among the age group is lower than in older groups.
Pfizer's vaccine was the first to be authorized for emergency use in the United States in December last year for those age 16 and older and was granted full U.S. approval in August. Earlier this week, Moderna reported interim data showing that its vaccine generated a strong immune response in children ages 6 to 11 years. It is awaiting a U.S. regulatory decision on the authorization for children between ages 12 and 17.